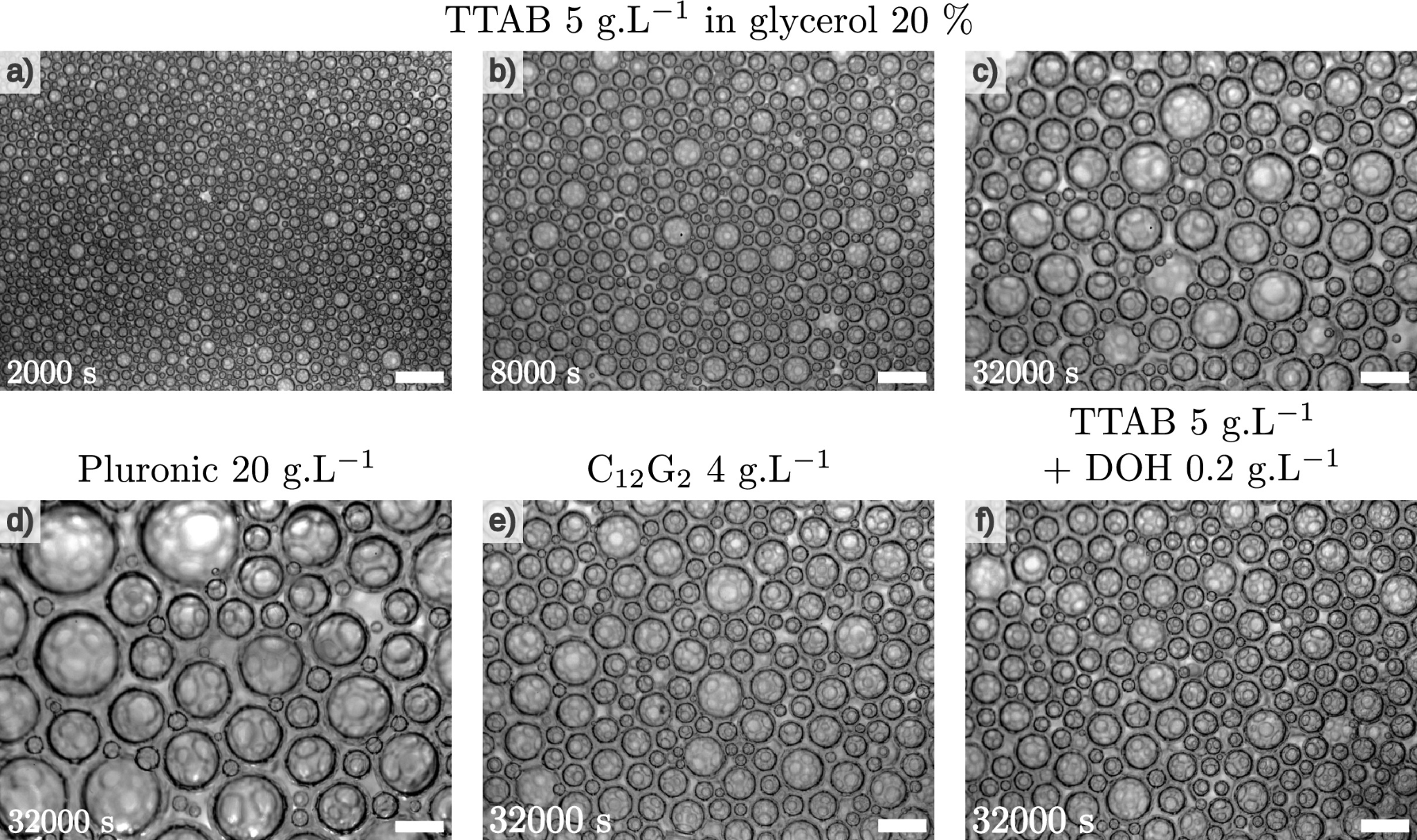
| In a long-term study carried out on the International Space Station (ISS), researchers were able to study precisely the physico-chemical factors influencing the long-term stability of foams by avoiding gravity-induced drainage. |
QWhether in a cappuccino, shaving foam or a fire extinguisher, foams are part of our everyday lives. Unfortunately, as they are made up of liquid and gas, they are intrinsically unstable. On Earth, they evolve rapidly as the liquid drains away. The most stable foams survive this initial evolution, but even once most of the drainage is complete, their bubbles continue to grow, either by merging or by a mechanism known as ripening: the small bubbles shrink and disappear while the larger ones grow, the gas diffusing from one to the other through the liquid phase. These subsequent phases of evolution depend in a complex way on the fairly fine physico-chemical characteristics of the mixture making up the foaming liquid, which explains why even today, the general prediction of the stability time of a given foam as a function of its composition remains an open question.
The present study was carried out in the following CNRS laboratories:
- Laboratoire de Physique des Solides (LPS, CNRS / Université Paris-Saclay)
- Institut des NanoSciences de Paris (INSP, CNRS/Sorbonne Université)
- Laboratoire Navier (NAVIER, CNRS/École Nationale des Ponts et Chaussées/Université Gustave Eiffel)
- Sciences et Ingénierie de la Matière Molle (SIMM, CNRS/ESPCI Paris-PSL/Sorbonne Université)
Understanding and slowing down these phenomena is essential to improving the performance of industrial foams. One obstacle to progress is that on the ground, gravity-induced drainage disturbs measurements by creating an inhomogeneous liquid distribution. To observe curing without interference, a consortium of researchers carried out observations of the evolution of foams generated on board the International Space Station, an environment with zero effective gravity. They were able to compare the stability of very liquid foams (known as “wet foams”) prepared with nine different stabilising agents – conventional surfactants, a polymer and a food protein. To do this, they took extremely precise measurements of the speed at which the bubbles grew and showed that this varied by a factor of 10 depending on the chemical nature of the stabiliser used. Until now, specialists in the field thought that this speed depended mainly on the permeability of the interstitial liquid (i.e. the capacity of liquid films to let gas through), but the researchers discovered that two other key factors come into play. Firstly, the adhesion between the bubbles, which influences their local structure, is important when the liquid fraction is not too high. Secondly, diffusion of the gas out of the films, via the capillary bridges or ‘Plateau edges’ (which always join at the intersection of three films and make up the bulk of the foam’s liquid ‘skeleton’), proves decisive in cases where the permeability of the very fine interfaces is particularly low. These effects can either accelerate or slow down the ripening of the foam, which illustrates the complexity of the overall phenomenon, a complexity that this work finally makes it possible to rationalise by simultaneously taking into account the interfacial properties and structural characteristics of the foams studied.
In conclusion, this work provides a better understanding of the role of surfactant chemistry in the curing of wet foams. It opens up prospects for designing foams in a variety of fields, from cosmetics to pollution control, via innovative materials. These results are published in the Journal of Colloid and Interface Science.
References :
Wet foam coarsening: More than film permeability, Alice Requier, Shailesh Varade, Nicolò Galvani, Marina Pasquet, Sylvie Cohen Addad, Cécile Gehin-Delval, Reinhard Höhler, Olivier Pitois, Emmanuelle Rio, Anniina Salonen, Dominique Langevin, Journal of Colloid and Interface Science, 696, 137825 – Published 12 may 2025.
Doi : 10.1016/j.jcis.2025.137825
Contact :
Dominique Langevin, CNRS Senior researcher at The Solid State Physics Laboratory (LPS) : langevin@lps.u-psud.fr
Communication CNRS Physique, cnrs-physique.communication@cnrs.fr
