Frozen water can take on up to three forms at the same time when it melts: liquid, ice and gas. This principle, which states that many substances can occur in up to three phases coexisting simultaneously, was explained 150 years ago by the Gibbs phase rule. Today, researchers from Eindhoven University of Technology (the Netherlands) and the LPS have pushed the limits of this classical theory, by demonstrating the possibility of a five-phase equilibrium in a simple binary mixture, something that many scholars considered impossible. This study provides useful insights into controlling the phase behaviour of complex nanoparticle mixtures that are at the basis of many food products, paint or optical displays.
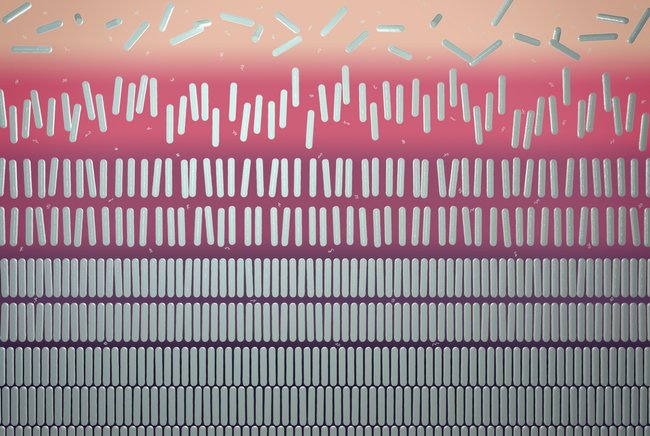
A five-phase equilibrium with at the top a gas phase with unaligned rods (isotropic phase), then a liquid phase with rods pointing in about the same direction (nematic liquid crystal), subsequently a liquid phase with rods lying in different layers (smectic liquid crystal), and two crystalline solid phases at the bottom. Image: ICMS animation studio.
The founder of contemporary thermodynamics and physical chemistry is the American physicist Josiah Willard Gibbs. In the 1870s he derived the phase rule, which describes the maximum number of different phases a substance or mixture of substances can assume simultaneously. For pure substances, the Gibbs Phase Rule predicts a maximum of 3 phases (referred to as a triple point).
The researchers analysed a simple theoretical model for a binary mixture of two colloidal substances, rods and polymers, immersed in a background solvent. While rod interactions are purely repulsive, the presence of the polymer imparts subtle, tunable attractive forces between the rods.
According to Gibbs’ phase rule, the binary mixture studied by the researchers would exhibit a maximum of only three phases coexisting at one specific point. But Tuinier and his colleagues have demonstrated that, under certain circumstances, four phases may exist simultaneously. There is even one point at which there are five coexisting phases. Two too many, according to Gibbs. At this quintuple point, a gas phase, two liquid crystal phases, and two solid phases with ’ordinary’ crystals exist simultaneously (see Figure). The crux lies in the shape of the particles in the mixture. Gibbs did not take this into consideration, but this study demonstrates that it is the specific dimensions of the particles play a major role. In addition to the known thermodynamic variables temperature and pressure, one obtains two additional shape-related variables: the length of the rod in relation to its diameter, and the diameter of the rod in relation to the diameter of the polymer also present in the solution.
Broadening our fundamental knowledge about phase transition in mixed colloidal systems will lead to more accurate predictions and control over when these kinds of transition occur. Implications in real life are manifold. One could think of pumping complex mixtures around industrial reactors, or the production of complex formulations based on colloidal mixtures such as dairy products, paint, optical displays and smart windows.
Reference
Defying the Gibbs Phase Rule: Evidence for an Entropy-Driven Quintuple Point in Colloid-Polymer Mixtures
V. F. D. Peters, M. Vis, Á. González García, H. H. Wensink, and R. Tuinier Physical Review Letters 125, 127803 (2020)
doi:10.1103/PhysRevLett.125.127803
