In collaboration with researchers from the Institut de Biologie Intégrative de la Cellule, the SOLEIL synchrotron and the University of California, Riverside, a team from the Laboratoire de Physique des Solides has shed light on the self-assembly process of the hepatitis B virus capsid in the presence of two types of antiviral. They have shown that the deformation of the normally icosahedral capsid towards ellipsoidal or even aberrant morphologies is due to the alteration of interaction and curvature energies induced by the antivirals.
More than 292 million people are chronically infected with the hepatitis B virus (HBV), mainly in Africa and South Asia, due to the lack of systematic vaccination. HBV causes severe pathologies such as liver cirrhosis and hepatocellular carcinoma, sometimes leading to the death of almost a million patients. HBV is an enveloped virus consisting of a protein shell – the capsid – arranged in an icosahedral symmetrical structure protecting the genome in the form of DNA. During a life cycle, the capsid self-assembles inside the host cell. Antiviral molecules, known as assembly modulators, have been developed to target capsid proteins and disrupt the self-assembly process. However, the kinetic pathways and physical mechanisms of action of these modulators have not yet been explored.
Using time-resolved small-angle scattering at the SOLEIL synchrotron, we demonstrated the acceleration of capsid self-assembly kinetics induced by the presence of modulators. Using a phase transition relaxation model, we estimated that the effective interaction energy between proteins varied from 9 kBT – kBT being the thermal energy – in the absence of modulator, to more than 18 kBT in modulator superstoichiometry. This induces an increase in capsid stretching energy, resulting in a profound change in morphology. While the native capsid is icosahedral and comprises 240 proteins, our transmission electron cryomicroscopy observations revealed capsids that were either slightly elongated or aberrant with a twofold increase in size. The important parameter turned out to be the Föppl-von Kármán number, i.e. the ratio of stretching energy to bending energy, which, through coarse-grained Monte Carlo simulations, enabled us to reproduce the observed morphologies (Figure). This work sheds new light on the action of antiviral molecules, which could be used to modulate the morphology of biomimetic nanocapsules derived from viral capsids.
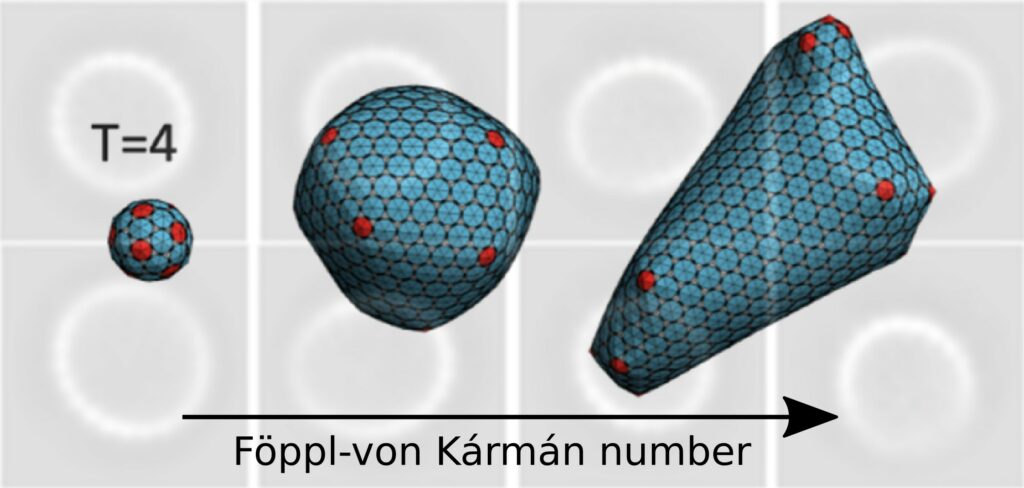
Figure. Monte Carlo simulations illustrating the change in capsid morphology by altering its elastic properties via the Föppl-von Kármán number. The bottom image shows capsid class averages in the presence of modulators observed by transmission electron cryomicroscopy.
Référence
Energetics and Kinetic Assembly Pathways of Hepatitis B Virus Capsids in the Presence of Antivirals
K. Kra, S. Li, L. Gargowitsch, J. Degrouard, J. Pérez, R. Zandi, S. Bressanelli, G. Tresset,
ACS Nano, 2023, 17, 12723-12733
doi: 10.1021/acsnano.3c03595
To find out more
Read the full story on the SOLEIL synchrotron website
This news was highlighted at the CNRS Institut of Physics (in french)
Contact
Guillaume Tresset
