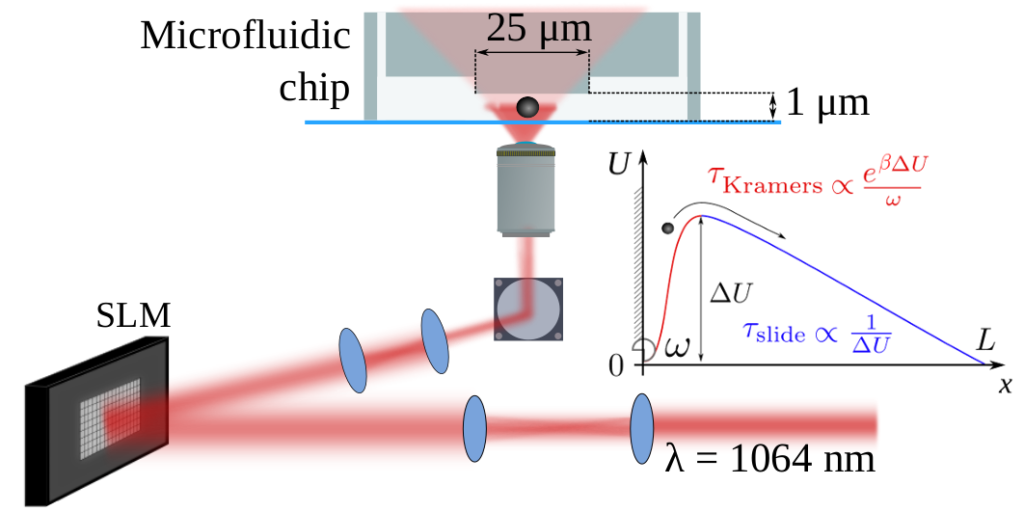
In physics, chemistry or biology, reaction rates are very often limited by energy barriers that have to be overcome by thermal activation. Researchers from LPS, LPTMS (CNRS/U. Paris Saclay) and the University of Cambridge demonstrated that we can play on the profile of a barrier to accelerate the crossing: surprisingly, the optimization of profiles leads to higher energy barriers.
Arrhenius’ law (1889) is a cornerstone in our understanding of many kinetic processes in chemistry, thermodynamics or molecular biology. This empirical law stipulates that reaction rates depend exponentially on the energy barrier which separates the reactants from the final products; this is the so-called activation energy. A satisfactory theory was proposed 50 years later by Kramers for Brownian systems. Kramers’ relationship introduces the coupling to the environment (the suspending fluid) via a friction parameter, and confirms the exponential relationship between reaction speed and activation energy. It is therefore natural to think that the higher the activation barrier, the slower it is to cross, and therefore to conclude that the absence of the activation barrier would lead to maximum reaction rates. A collaboration between the LPS Orsay and the LPTMS, as well as the Cavendish laboratory in Cambridge, has shown that this intuition based on the relationships of Kramers / Arrhenius was incorrect. In other words, it is not the free diffusion and therefore the absence of any barrier that minimises the time of first passage at a target point for a Brownian particle. The very existence of a barrier speeds up the process!
Contact
References and links
Optimizing Brownian escape rates by potential shaping
M. Chupeau, J. Gladrow, A. Chepelianski, U. Keyser, E. Trizac
Proc Natl Acad Sci (USA) 117, no. 3, 1383 (2020)
doi:10.1073/pnas.1910677116
