In some conditions, very elongated rod-like silver nanoparticles can be grown on gold seeds. What is the origin of this surprising shape? LPS researchers, in collaboration with the MPQ laboratory and the SOLEIL synchrotron (SWING beamline) studied in real time the growth in solution of silver nanorods onto a gold core by the combination of three in situ techniques. Their multiscale approach revealed an unexpected role for ascorbic acid, a reducing agent widely employed for nanoparticle synthesis, in the formation of anisotropic nanostructures.
Controlling the size and shape of metallic nanoparticles is one of the main goals of materials science. To achieve it, one must identify the respective roles of the molecular species involved. Such an analysis is difficult, on the one hand because each technique only yields incomplete or indirect information on the synthesis, and on the other hand because each reagent can play several roles in this process.
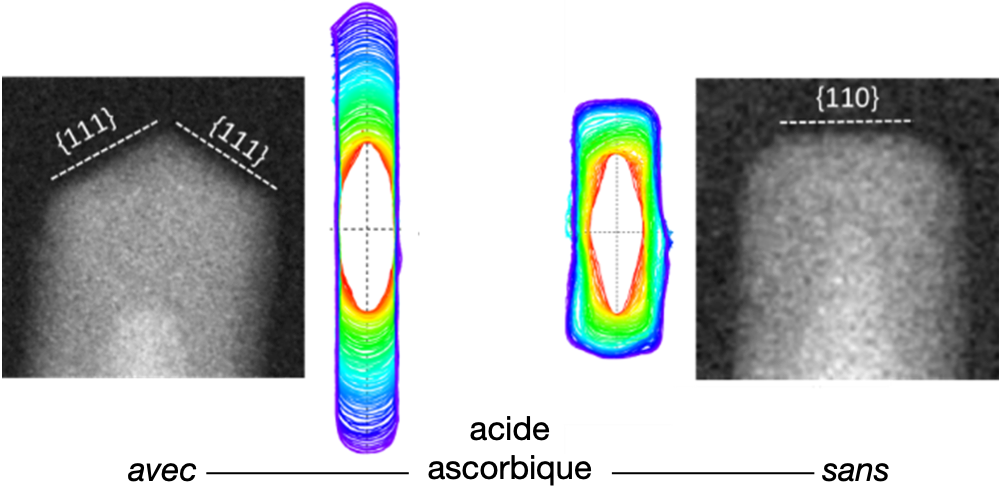
LPS researchers, in collaboration with the Matériaux et Phénomènes Quantiques laboratory (MPQ, CNRS/Université de Paris) and with the SWING beamline of the SOLEIL synchrotron combined three in situ techniques allowing to probe in real time the growth of nanostructures in solution: UV-Visible absorbance spectroscopy, small-angle X-ray scattering and transmission electron microscopy in liquid cell.
In this work, the researchers studied the growth of silver nanorods onto bipyramid-shaped gold seeds and revealed the origin of the elongated shape of these objects. Until now, the consensus in the research community explained it by the conjunction of twinning in the gold seeds with the preferential adsorption of halide ions, present in the solution, onto certain crystal facets. The new study demonstrated that ascorbic acid, used as reducing agent, is in fact indispensable to the anisotropic growth, acting in synergy with the halides to stabilize particular facets of the silver crystal. This collaborative endeavour illustrates the strength of in situ correlative strategies in rationalizing the chemical synthesis of nanomaterials. https://widgets.figshare.com/articles/12040650/embed?show_title=0
Reference
Real-time in situ observations reveal a double role for ascorbic acid in the anisotropic growth of silver on gold
Kinanti Aliyah, Jieli Lyu, Claire Goldmann, Thomas Bizien, Cyrille Hamon, Damien Alloyeau, et Doru Constantin
Journal of Physical Chemistry Letters 11(8), 2830-2837 (2020)
DOI:10.1021/acs.jpclett.0c00121
